The ATOMIC study: atezolizumab plus FOLFOX chemotherapy significantly improves disease-free survival and reduces the risk of recurrence in stage III colon cancer
A new study – the ATOMIC trial – has found that adding the immunotherapy atezolizumab to standard chemotherapy (FOLFOX) significantly improves outcomes for people with stage III deficient DNA mismatch repair (dMMR) colon tumours – a type of cancer that makes up about 10–15% of non-metastatic colorectal cancers.
Key Findings
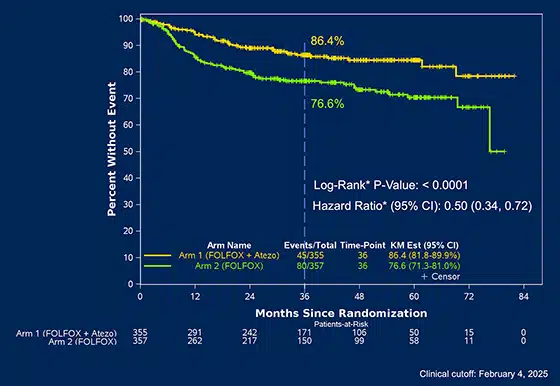
- 50% lower risk of cancer recurrence or death when atezolizumab is added to chemotherapy compared to chemotherapy alone.
- Disease-free survival (DFS) at 3 years:
- With atezolizumab + FOLFOX: 86.4%
- With FOLFOX alone: 76.6%
- Benefits were seen across all age groups, genders, and tumor characteristics.
- Overall survival data are still being collected.
Side Effects
- More high-grade side effects occurred with the combination treatment, including:
- Fatigue
- Nausea
- Loss of appetite
- Low white blood cell counts
- These side effects were generally manageable and similar to what is expected with immunotherapy and chemotherapy.
Why This Matters
- This is the first large study showing that adding immunotherapy after surgery can help prevent colon cancer from returning in patients with dMMR tumors.
- Experts believe this approach may soon become a new standard of care for this group.
- The results are important for people with Lynch syndrome and those with sporadic dMMR colon cancers.